Information & Impacts
Here are some examples of our research and the impact it has on companion animal health

Labrador retrievers with myopia to benefit from genetic study
Dogs are known for their excellent sense of smell. Their vision, on the other hand, isn’t the best. While dogs may have a broader view because of the positioning of their eyes, they also have a limited depth of field, meaning they can’t focus on images at far distances. Compounding that limitation, some dogs also suffer from an inherited condition called myopia, or near-sightedness.
Working dogs affected with myopia aren’t able to perform visually to the same degree as unaffected dogs. A service dog with myopia might struggle to guide a visually impaired person. A dog that usually helps with police work might have to stop. Other dogs might be unresponsive to off-leash commands like “come” or “stay” if their human companion stands too far away. The dog could grow confused and agitated.
Myopia affects about 15 percent of Labrador retrievers, which have been identified to have myopia attributable to an increase in the axial length of the vitreous chamber of the eye (the length of the main chamber in the eye). This is also the most prevalent cause of human myopia. Researchers with the Genetics Program at the CCAH are currently conducting a genetic investigation of myopia in Labrador retrievers to identify the region of the dog genome associated with this condition by collecting DNA samples (blood) and noninvasive measurements of the refractive state (where the eye is focused). The project is being led by doctors Ann Strom and Christopher Murphy, both veterinary ophthalmologists in the school's Ophthalmology Service, as well as Dr. Danika Bannasch, director of the CCAH Genetics Program.
Knowing the genetic cause of myopia will help scientists develop both a genetic test for this condition and determine improved treatment options. The current test performed for myopia in dogs is called “streak retinoscopy,” but this technique can be challenging to learn and most veterinarians are not trained to practice this technique. UC Davis researchers think a genetic test would offer a much better diagnostic method, which would allow veterinarians to deliver improved clinical services and treatment.
"There is really no good treatment at the moment other than placing prescription contact lenses or "doggles" (dog glasses)," Strom says. “Laser treatment for this problem isn’t currently used in dogs. If a gene for the condition is found in dogs, a gene test can be developed and dogs can be screened prior to breeding and excluded from breeding if they are affected or carriers. Gene therapy may also be a possibility in the future." As part of this research, a dog’s entire genome will be evaluated for an association with myopia. The study now includes 235 Labrador retrievers and is funded with a CCAH grant.
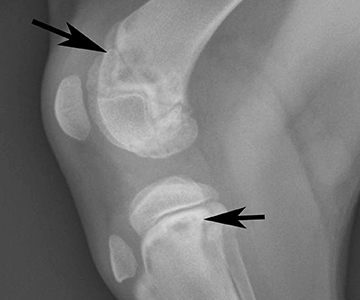
Finding the gene responsible for hypertrophic osteodystrophy in dogs
Puppies affected with canine hypertrophic osteodystrophy (HOD), a developmental disease, suffer musculoskeletal pain and often refuse to walk or even stand. They experience lameness, usually along with fever, lethargy and loss of appetite. Sometimes these dogs experience diarrhea, puppy acne and discharge from the eyes and nose.
In Weimaraners, HOD is often severe and sick puppies may have several episodes until complete closure of the growth plates, which usually requires hospitalization for intensive care. The harsh pain and poor quality of life, accompanied by the high costs of hospitalization, leads some owners to elect euthanasia.
In 2007, veterinarian Dr. Noa Safra completed her PhD at the UC Davis School of Veterinary Medicine. Since then, she’s immersed herself in a quest to understand the genetic cause of various diseases affecting dogs. A recent pursuit involves finding the gene responsible for HOD in Weimaraners and other susceptible breeds - rapidly growing, large dogs such as the Great Dane, Boxer, German shepherd, Labrador retriever and Irish setter - between eight weeks and eight months of age.
Veterinarians currently diagnosis HOD through X-rays and treat the disease with what’s called “supportive care,” which refers to measures that control or relieve signs and side effects to improve comfort and quality of life. HOD is a disease of growing puppies; when they reach adulthood, most dogs stop having episodes. But some dogs continue to suffer from different degrees of illness - mainly fever and malaise - as adults.
Safra -- a lover of all dogs, especially Weimaraners, which she breeds and has had as pets for many years -- has been leading the HOD research at the Bannasch Genetics Laboratory in the CCAH. Her study, initiated when she was a post-doctoral fellow, has so far enrolled about 300 dogs of various breeds affected by HOD.
Previously, Safra’s appreciation for Weimaraners motivated her search to find the gene involved in spinal dysraphism — a neural tube defect that affects a very small percentage of this dog breed. She published her findings in the scientific journal PLOS Genetics in 2013. Puppies with the disorder are unable to move their rear legs normally, and can’t walk or run. “They hope like bunnies, but most of them get around very well, and fast,” Safra says. They drag themselves using their front end. “This is a non-progressive disorder, meaning that while it doesn’t get better it doesn’t get worse, either,” Safra says. “Even the most severely affected puppies make perfect pets. They move and they play and they’re happy, and they don’t suffer pain. So it’s mostly just a very large deviation from normal dog movement.”
Although Safra is now a resident in Clinical Pathology at the school, her project on HOD will continue on at the school.
- TOP -
 Eliminating juvenile-onset Addison’s disease
Eliminating juvenile-onset Addison’s disease
What if your dog had a potentially fatal inherited disease that could be treated with supplemental hormones? This solution sounds simple enough. All you’d need is a genetic test to look for the mutation causing this particular disease.
Thanks to researchers in the Bannasch Laboratory at the CCAH, a genetic test is now available for juvenile-onset Addison’s disease in the Nova Scotia Duck Tolling Retriever dog breed, or “Toller” for short. This inherited endocrine disease is caused by an autoimmune attack of the adrenal glands, resulting in decreased production of vital hormones. While possibly fatal, the disease can typically be managed by administering the missing hormones to the dog (although this can be time and money intensive for owners).
Understanding Addison’s disease is one of the major, long-term projects at the laboratory, run by Dr. Danika Bannasch, which seeks to find the genetic basis for diseases in pets and other animals. In terms of Addison’s disease, the laboratory focuses on Tollers because of the breed’s increased incidence compared to the general dog population, according to a survey from the early 2000s.
Emily Brown, a DVM PhD student in the lab who is researching Addison’s disease for her thesis, says studying genetic diseases in pure breeds is helpful because they come from a small number of founding dogs, which makes gene mapping an easier process. But Addison’s disease is still complicated. Dogs have been diagnosed as early as 3 weeks of age and as old as 11 years. The clinical signs of the disease can be non-specific, such as vomiting, diarrhea, decreased appetite, weight loss and slow heart rate. There’s nothing that directly points to Addison’s disease, so veterinarians must run blood tests to measure levels of sodium, potassium and cortisol to get a diagnosis.
Developing the genetic test for juvenile-onset Addison’s disease has helped Toller breeders start to eliminate this condition from the population. Our researchers are still working to identify the mutation responsible for adult-onset Addison’s in Tollers.
This disease also affects humans; in our bodies, the potentially life-threatening disease has many mutations, making it especially hard to genetically test for. Studying this autoimmune disorder in canines may one day translate to people. “One thing often overlooked is the value of a dog as a human animal model,” Brown says. “Auto-immune disorders affect 5 to 10 percent of people in the industrial world, so this could also help with human patients.”
- TOP -
 Researchers use ancient dog DNA to understand their human companions
Researchers use ancient dog DNA to understand their human companions
Around 1,000 years ago, a major demographic upheaval began to occur in the North American Arctic. The Paleo-Eskimo, ancient people who had survived in isolation for more than 4,000 years, started to die off. By 300 years later, they were gone.
The Paleo-Eskimos were the first humans to inhabit this unforgiving landscape of today’s Greenland, Alaska and Canada. Then the technologically advanced Thule — ancestors of the present-day Inuit — arrived. But what explains the die-off of the Paleo people and what was their relationship to other indigenous populations? Our researchers are using the ancient DNA of Arctic dogs to understand what these remains imply about the interactions among their human companions.
Over the course of the past 6,000 years that humans have occupied the North America Arctic, these cultures increasingly depended on dogs for hunting, hauling, protection and transportation, according to Dr. Sarah Brown, a National Science Foundation-funded post-doctoral scholar who started her research at UC Davis and is now based at UC Merced. She designed her research to address questions about the timing and spread of dogs from Asia to Alaska, and Canada and Greenland, and how local breeds developed.
“Our results preliminarily show that dogs’ patterns are similar to what is seen in the human data — that Paleo-Eskimo and Thule dogs were unrelated and are of different migration waves,” Brown says. “This is exciting, as it suggests that dogs might be a good way to retrace the migration history of humans.”
Our researchers collected ancient DNA from Arctic dogs in collaboration with UC Davis archeologist Dr. Christyann Darwent who has ongoing evacuations in Alaska and Greenland (she is the principal investigator on a National Science Foundation grant). Other samples came from the collections of museums in the United States, Canada and Denmark. Researchers generated full mitochondrial genomes for about 100 individual dogs dated between around 4,000 and 1,000 years old and scattered throughout the Arctic.
“We can use DNA to reconstruct the demographic past. Looking for the genetic footprint of the population, the expansion and contraction, helps us understand how dogs spread across the Arctic,” says Dr. Benjamin Sacks, associate adjunct professor in the Veterinary Genetics Laboratory. Sacks is based at CCAH, and serves as the faculty co-principal investigator for the project.
Brown says the final results — as yet unpublished — will, among other things, contribute to anthropological and archaeological understanding of the movements and cultural interactions of the region’s indigenous peoples.
- TOP -

Gene mutation for heart disease in Newfoundland dogs identified
Newfoundlands — those massive, furry, black dogs — have captured many a heart with their hallmark size, sweet nature and loyalty. Unfortunately, these gentle giants’ own hearts are all too often afflicted with a potentially lethal congenital disease called subvalvular aortic stenosis (SAS).
SAS is the most common inherited heart disease in dogs — but it is far more prevalent among Newfoundlands than any other breed. But now, thanks to a groundbreaking new study, that may be changing. Recently, a team of veterinary researchers — led by Dr. Josh Stern, chief of the UC Davis veterinary hospital’s cardiology service — discovered a genetic mutation responsible for SAS in Newfoundland dogs. This discovery has the potential to help breeders and owners eradicate this deadly disease from Newfoundland breeding lines.
SAS causes an abnormal ring or ridge of tissue to form below the heart’s aortic valve, making it difficult for blood to pump out of the heart and around the body. Diagnosing and treating SAS, however, is particularly challenging because the disease can vary greatly in severity. Dogs with the severe form are likely to die before they are 4 1/2 years old, even with therapeutic drugs. But dogs with the mild form can life a completely normal lifespan, which means they often go undiagnosed. “Unfortunately, these dogs may get bred and propagate this lesion within the population,” Stern says.
Veterinarians may discover the disease when they detect a heart murmur and conduct further diagnostic tests such as chest X-rays, an echocardiogram or an electrocardiogram. But often the first sign that a dog has SAS may be a collapse, fainting spell or even sudden death.
The discovery of the SAS mutation in Newfoundlands didn’t happen overnight. Stern started collecting DNA samples from Newfoundlands a decade ago, as a veterinary student at The Ohio State University. “I started looking at Newfoundland pedigrees and trying to piece together how this disease was inherited,” he explains. Eventually he began to try to identify a mutation responsible for the disease.
Researchers began by conducting a “whole genome” analysis, scanning thousands of genes for abnormalities, which revealed that the mutation associated with SAS resides in a gene called PICALM. This same mutation has been linked to the formation of plaque-like lesions in the brains of people with Alzheimer’s disease. This study led to a DNA-based test to screen Newfoundland dogs for the mutation. “Our hope now is that breeders will be able to make informed breeding decisions and avoid breeding dogs that harbor the mutation, thus gradually eliminating the disease from the Newfoundland breed,” Stern says. The test is available through the school’s Veterinary Genetics Laboratory.
Our researchers are continuing to unravel the mysteries of PICALM in order to eventually develop novel treatment options for those dogs that are severely affected by SAS. They are also studying why SAS is mild in some dogs while causing severe symptoms in others, as well as trying to find the mutations that cause the disease in six other breeds, with the help of seed funding from the Center for Companion Animal Health.
Their findings have implications for the study and treatment of SAS in human children. Because the genetic basis of the disease in people is not yet fully understood, knowledge of this gene may be really important for researchers in human medicine.
- TOP -

Our vets are pioneers in veterinary genetics
Genetics testing and research are vibrant, cutting-edge components of the UC Davis School of Veterinary Medicine. Genes, environment, and their interaction play critical roles in animal health and disease, and our scientists are continuously breaking new ground and deepening our understanding of the animal world through advancements in this exciting field.
Genetics research not only furthers understanding of which animals are at risk for particular diseases, but ongoing discoveries can also lead to the development of diagnostic tests and potential gene therapies, resulting in earlier disease intervention and improved health outcomes.
The recent mapping of horse, dog and cat genomes has made available powerful tools for the investigation of inherited diseases; it also empowers researchers to apply this vast new knowledge toward correcting genetic defects and disease susceptibilities. Each research discovery accelerates our understanding of animal diseases, which in turn can provide vital information about related human diseases.
Yellow Fever in Bolivian Howler Monkeys
Veterinary scientists helped identify and respond to a Yellow Fever outbreak in 2012 after five howler monkey carcasses were found near a wildlife sanctuary in eastern Bolivia. PCR tests revealed that the monkeys were infected by a flavivirus. The Ministry of Public Health was immediately notified and cDNA sequencing confirmed that the infections had been caused by two Yellow Fever viral strains, both of which were related to human cases. Only eight days passed between the onset of outbreak and notification of the Bolivian government. Preventive measures were promptly implemented in the affected area, including vaccination campaigns, public outreach and mosquito control. Thanks in part to the fast response, no human cases occurred during the outbreak.
Discovering a new virus
UC veterinary wildlife epidemiologists, with a team of international researchers, discovered a new SARS-like coronavirus in Chinese horseshoe bats that can be directly transmitted to humans. The virus is closely related to the SARS coronavirus that erupted in Asia in 2002 and 2003 and caused a global pandemic crisis. These findings open doors for scientists to conduct detailed studies to create control measures to thwart outbreaks and provide opportunities for vaccine development; and highlight the importance of research programs such as UC Davis’ PREDICT, which is actively building a global surveillance system to detect and prevent spillover of pathogens of pandemic potential that can move between wildlife and people.
Heart disease in cats
Veterinary cardiologists identified the gene mutation responsible for hypertrophic cardiomyopathy, the most common cause of heart disease in cats. This inherited disease is also important in humans and is frequently responsible for sudden cardiac death. The discovery marks the first report of an identified spontaneous genetic mutation causing heart disease in a cat or dog. The findings paved the way for development of a screening test that identifies cats carrying this genetic mutation so that they can be identified before they are bred, thus reducing or eliminating the incidence of the disease. As they continue to study this disease and mutation in cats, researchers are also hopeful that the discovery will provide a valuable model for investigators in human medicine who are studying the disease.
Gene mutation in dogs offers clues for neural tube defects in humans
Veterinary scientists identified a gene related to neural tube defects in dogs. The researchers also found evidence that the gene may be an important risk factor for human neural tube defects, which affect more than 300,000 babies born each year around the world.
Cleft palate discovery
Veterinary geneticists identified the genetic mutation responsible for a form of cleft palate in the dog breed Nova Scotia Duck Tolling Retrievers. They hope that the discovery, which provides the first dog model for the craniofacial defect, will lead to a better understanding of cleft palate in humans.
Avian Responses to West Nile Virus
Veterinary pathologists have a number of studies under way to assess the effects of West Nile virus on certain species of birds. The team has developed molecular tools specific for yellowbilled magpies and is comparing magpie DNA collected before West Nile virus entered California with samples collected recently. They are also examining whether the virus is changing the population structure of crows, Swainson's hawks and red-tailed hawks statewide. Knowledge gained from these studies benefits human health, as these birds are particularly susceptible to the disease and are excellent early-warning sentinels for virus activity.
Raccoons may provide cancer clues
Scientists at the School of Veterinary Medicine and the California Animal Health and Food Safety Laboratory discovered a previously unidentified virus found in rare brain tumors emerging among raccoons in Northern California and Oregon. Dubbed raccoon polyomavirus, researchers suspect this virus contributes to tumor formation. Their findings could lead to a better understanding of how viruses can cause cancer in animals and humans.
Advances in Dalmatian health
A gene mutation that causes high levels of uric acid in all Dalmatian dogs and bladder stones in some Dalmatians has been identified by a team of researchers. The discovery equips dog breeders with the tools to eliminate that trait from the Dalmatian breed and yields clues to the cause of similar problems in humans.
Goats' milk a treatment for diarrhea
A veterinary professor led a study whose results showed that milk from goats that were genetically modified to produce higher levels of a human antimicrobial protein proved effective in treating diarrhea in young pigs The study offers hope that such milk may eventually help prevent human diarrheal diseases that each year claim the lives of 1.8 million children around the world and impair the physical and mental development of millions more. It also demonstrates the potential for food products from transgenic animals to one day benefit human health.
Increasing chicken production
Poultry experts are working in multi-disciplinary teams to dramatically increase chicken production among Africa's rural households and small farms. The new effort, called the Feed the Future Innovation Lab for Genomics to Improve Poultry, will identify genes crucial for breeding chickens that can tolerate hot climates and resist infectious diseases — specifically the devastating Newcastle disease, a global threat to food security whose economic impact is enormous.
Taking aim at foodborne diseases
The 100K Genome Project is an exciting and ambitious five-year effort to sequence the genomes of 100,000 infectious microorganisms, creating an enormous public database to reduce the typical public health response time to outbreaks from weeks to days using next-generation sequencing platforms. To date, the project has sequenced more than __ genomes, including pathogens responsible for common and debilitating foodborne infections such as Salmonella, Listeria, Campylobacter, and Vibrio. This important project will ultimately speed outbreak investigations, reduce illness, and facilitate the development of new rapid test methods to detect pathogens.
Vaccines target Rift Valley fever
Veterinary pathologists and others have developed two genetically engineered vaccines to combat the mosquito-borne Rift Valley fever, devastating to livestock in Africa and the Middle East. The virus also sickens people, who can be infected by mosquitoes or by direct contact with infected animals or their meat. Both new vaccines proved safe and produced significant immune responses in initial studies and scientists hope the new vaccines can be further developed for use in people.
Exploring insect vectors of human disease
The School’s Vector Genetics Laboratory focuses on the population and molecular genetics of insect vectors of human disease. Extensive fieldwork has been conducted in Mali, Cameroon, Brazil, Costa Rica and Nicaragua. One current project integrates vector population genomics, ecology and vector behavior with the goal of understanding the determinants of two mosquito behavioral phenotypes crucial to the transmission and control of malaria in Tanzania. Knowledge gleaned from the project will be vital for prediction of both possible downstream evolutionary responses to current vector control strategies, and also for the development of novel control strategies that improve the application of currently available vector control methods.
Human virus kills mountain gorillas
Researchers from the nonprofit Mountain Gorilla Veterinary Project and the Wildlife Health Center at UC Davis teamed up with others to discover that a virus that causes respiratory disease in humans has been linked to the deaths of wild mountain gorillas. The finding confirms that serious diseases can pass from people to these endangered animals; and that protected national parks do not provide a barrier against human diseases which can affect these primates.
Investigating Hummingbirds
Since 2010, the Hummingbird Health and Genetic Diversity Project has worked to determine levels of Hummingbird health and disease, evaluate migration ecology, and assess genetic diversity in California over large expanses of habitats. Very little is known about these small birds, and some California species are at severe risk of decline. Information gleaned from this project will help researchers to understand if populations are endangered and will assist in accurate monitoring for conservation.
Yosemite’s Great Gray Owl
Wildlife geneticists have documented Yosemite's Great Gray Owl as genetically distinct from the Great Gray Owl in other locations of western North America. DNA analysis is helping to fill significant gaps in the knowledge of the Great Gray Owl, and this genetic research has brought new attention to the species and added to the information necessary to formulate comprehensive and scientifically-defensible management and monitoring strategies for conservation.
The Mammalian Ecology and Conservation Unit
Part of the Veterinary Genetics Laboratory, conducts research that advances both the persistence of wild mammal biodiversity and our basic understanding of mammal evolution and ecology. Genetic tools are a cornerstone of the unit’s methodologies, and scientists also use field- and laboratory-based approaches in their research. Projects include:
- The exploration of the genetic basis and evolutionary origins of migratory behavior in black-tailed deer and Rocky Mountain mule deer
- Red fox genetics – researchers are using red fox samples to uncover the genetic relationship of red fox populations in different locations, which will answer questions such as if the population is genetically distinct and if it is relatively inbred or outbred. Results such as these can aid in understanding the evolutionary relationship between populations and how past climactic events have impacted modern populations.
Mountain Lion Health
The Wildlife Genetics, Genomics, and Population Health lab develops and applies the science of DNA analysis, disease investigation, and ecological tools to answer key questions toward improved population health, conservation, and management of wildlife birds and mammals.
- Using genetic tools, the lab studies the ecology and health of mountain lions as well as their populations of the past, present, and future. It has successfully developed puma-specific DNA markers and an extensive DNA data base, which allow the lab to detect and study kin relationships among mountain lions and to learn more about the mammals. These studies will help develop predictive models and project future trends for genetic diversity.
- A research team from the lab analyzed mountain lion DNA from trace samples collected at sites of livestock predation and public safety incidents. Genetic analysis was used to determine species, individual identity, and relatedness between individuals. This information will be useful to assess risk to human safety and manage human-lion interactions.
Black Bears linked to two colonies
Veterinary geneticists performed an analysis of DNA of 540 black bears across California, and discovered distinct population structure and genetic evidence of two historic colonization events. Molecular genetic techniques utilized in this study allowed historical reconstruction of anthropogenic events leading to changes in animal distributions.
- TOP -
